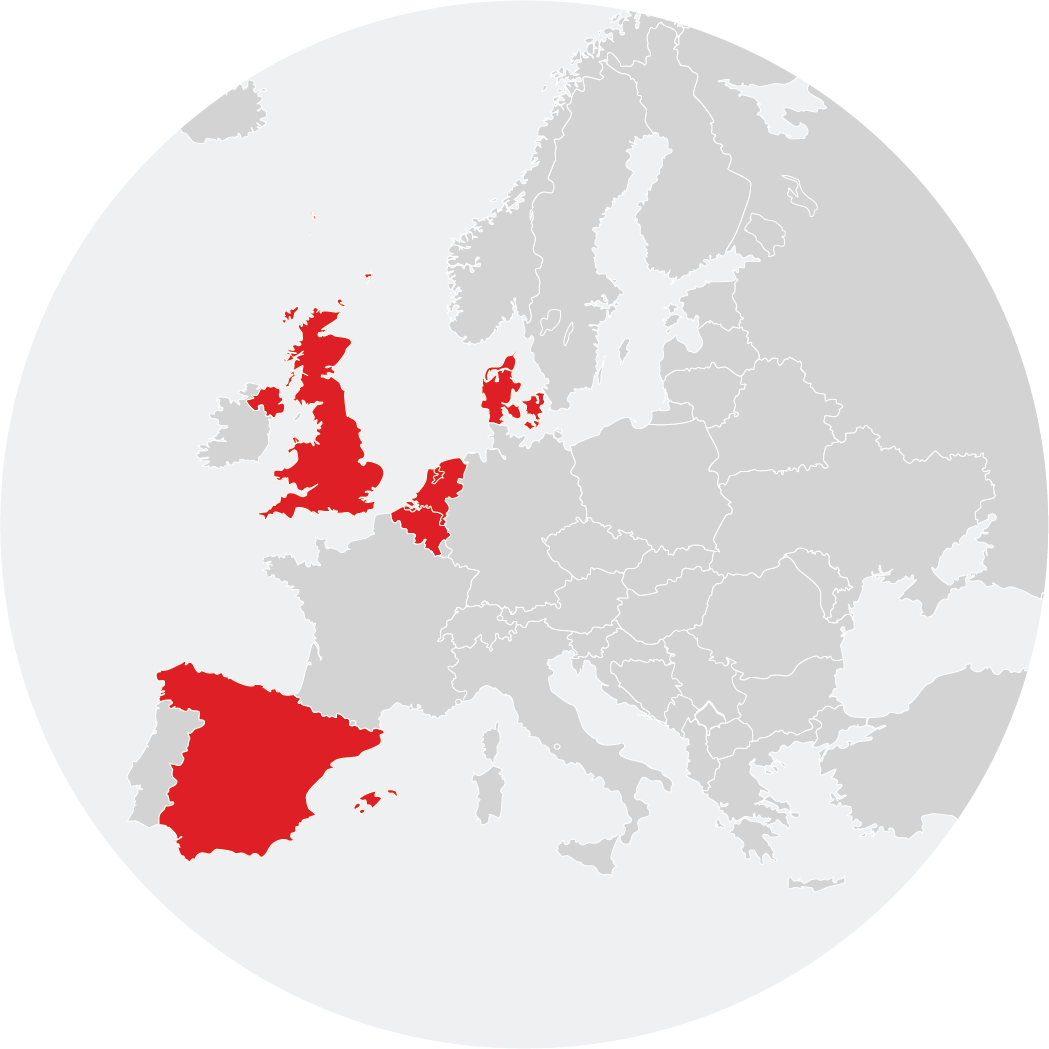
The EU-ADR Alliance represents an unprecedented collaboration framework to run a wide variety of studies based on a multi‐database approach to answer pharmacoepidemiological and drug safety questions. At the EU-ADR Alliance, we use routinely collected data extracted from multiple European privately and publicly owned Electronic Healthcare Records (EHR) databases. Our databases include about 20 million of active population, enabling us to access to broad range of data including paediatric.
Our Alliance provides a unique partnership of expertise, combined with a solid governance structure, and tested working methods. This allows us to run centralised studies and produce clinically meaningful results, thus generating valid and reliable evidence. Our team is made up of epidemiologists, physicians with active practice, pharmacists, biomedical scientists, statisticians, medical scientific advisers, data scientists and project managers. The studies are performed in a collaborative and federated manner at the request of external organisationsto run multinational databases studies.
Please see the EU-ADR Alliance latest publications
The EU-ADR Alliance builds on the results of the EU-ADR project “Exploring and Understanding Adverse Drug Reactions by Integrative Mining of Clinical Records and Biomedical Knowledge” (funded by the ICT Unit of the European Commission in the 7th Framework Programme (7FP), which represented a breakthrough in the use of technology to improve pharmacovigilance in federated studies. (Please see Coloma et al; EU-ADR Consortium. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011 Jan;20(1):1-11. doi: 10.1002/pds.2053. Epub 2010 Nov 8. PMID: 21182150).
For the latest EU-ADR Alliance publications, please click here
Several FP7 drug safety studies have successfully implemented the approach and tools developed EU-ADR:
– SOS, on the safety of non-steroidal anti-inflammatory drugs
– ARITMO, studying the arrhythmogenic potential of drugs
– SAFEGUARD, on the safety evaluation of adverse reactions in diabetes
The EU-ADR Alliance is an open and flexible federation of organisations based on the following principles:

We follow the The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP®) guide on methodological standards and Code of Conduct. This network coordinated by the European Medicines Agency (EMA) brings together public institutions and contract research organisations (CRO) who wish to investigate the safety of medicines, the benefits and risks of medicines, disease epidemiology and drug utilisation.
The ENCePP Code of Conduct establishes a set of rules and principles for pharmacoepidemiology and pharmacovigilance studies to promote transparency and scientific independence throughout the research process.
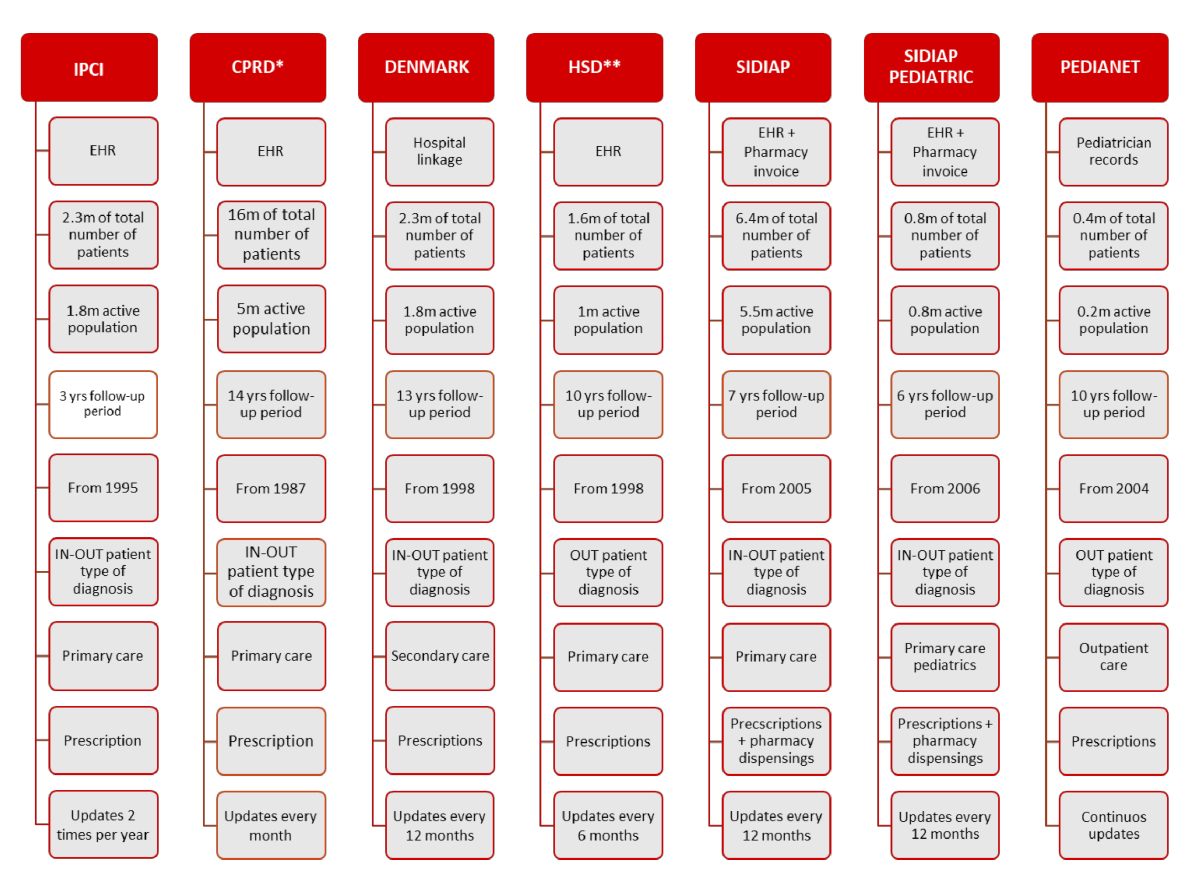
The EU-DR Alliance databases include about 20 million of active population, enabling us to access primary and secondary care, including both adult and paediatric health records.
* under license / **data refers to 800 general practitioners with fulfilled quality criteria for data registration
As a federated collaborative framework for observational studies, we:
The EU-ADR Alliance provides deep independent expertise and high standards in setting up and coordinating multi-country database studies including:

eu.adr.alliance@teamitresearch.com
Teamit Research
Barcelona Health Hub
Recinte Modernista de Sant Pau
c/ Sant Antoni María Claret, 167
08025 Barcelona (Spain)
T +34 930 180 163
I want to undertake a study. How do I start the process with EU-ADR Alliance?
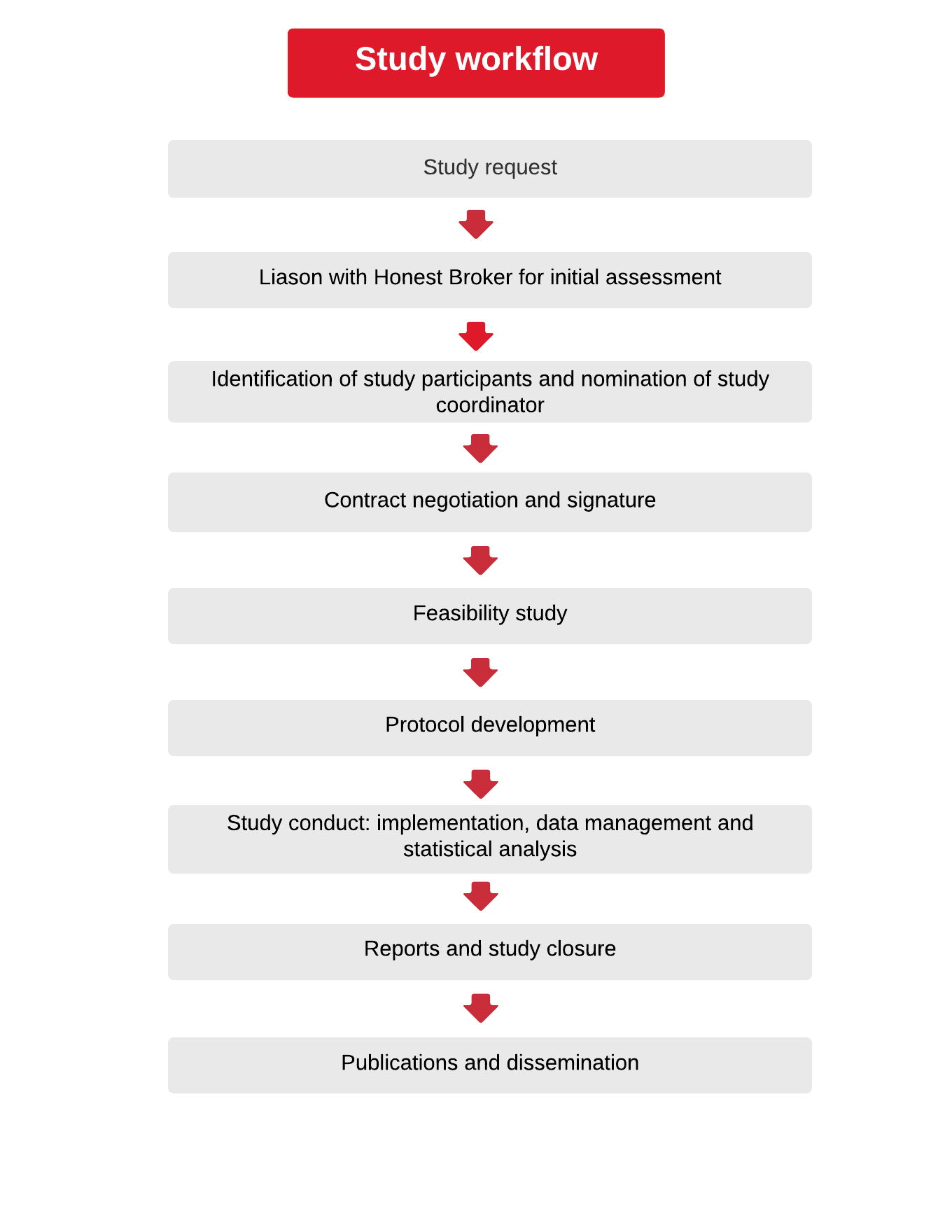
The first step is to contact a member of the EU-ADR Alliance with a brief study concept. The first point of contact during the early planning stages is usually Teamit Research, as this partner holds the role of “honest broker”. Please write to us. However, if you already know, or have worked with other partners in the Alliance, do not hesitate to make the first contact through that organization.
–
What is the EU-ADR Alliance procedure for choosing studies?
The Alliance partner that you first approached with the study concept will then share the information with the whole EU-ADR Alliance network. Any database may choose to participate or not in any study.
Once the partners confirm their interest in the study area, the most suitable Scientific Coordinator is agreed by all the partners involved. This will usually be the partner with the most experience in the therapeutic area of that specific study.
–
From the first contact, how soon can the EU-ADR Alliance start a new study?
This primarily depends on the study requester (sponsor). Once the EU-ADR Alliance partners decide that they are interested in undertaking a study, and the study concept is agreed upon with the sponsor, a contract can be drawn up. Once the contract is signed, the study can start.
My study may require European Medicines Agency (EMA) approval; how might this impact the study timeframe?
It can take 4 to 6 months to design a study protocol and get it fully approved by EMA. EU-ADR Alliance experience has shown that the preparation of the protocol for submission to EMA usually takes around 2 to 3 months. EMA then may take 2 additional months to carry out the review and request changes, or even reject the study.
–
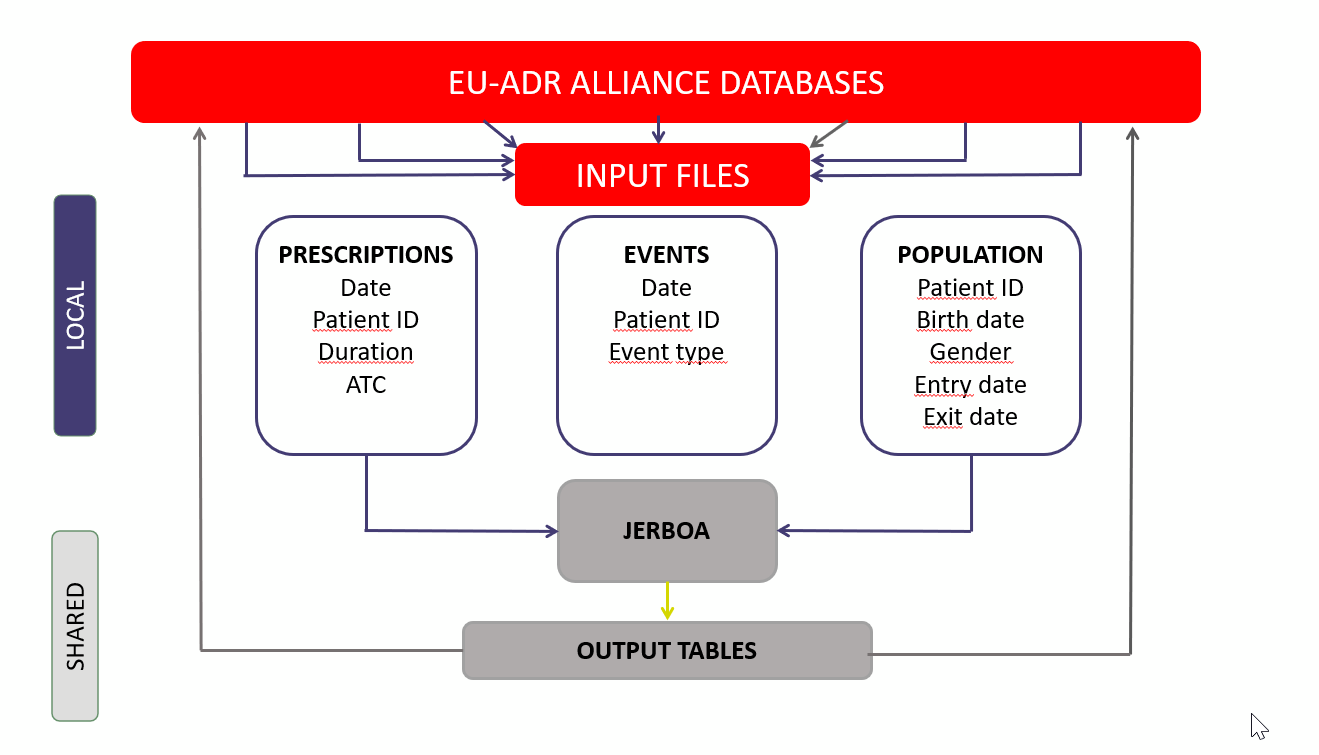
What is the process for data gathering and pooling?
Studies are selected by Alliance partners based on interest and whether they feel their data are suitable for the study objective(s). The Alliance partners then work on developing a common data protocol and locally create input files, which are then managed by a specific software called Jerboa, which aggregates the data and produces the desired output tables (e.g. incident rates of the event of interest, patterns of drug use) (See Coloma PM, Schuemie MJ, Trifirò G, Gini R, Herings R, Hippisley-Cox J, Mazzaglia G, Giaquinto C, Corrao G, Pedersen L, van der Lei J, Sturkenboom M; EU-ADR Consortium. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011 Jan;20(1):1-11. doi: 10.1002/pds.2053. Epub 2010 Nov 8. PMID: 21182150).