Experts in scientific management of pharmacoepidemiology research and regulatory science initiatives
At Teamit Institute, we:
Coordinate studies to ensure the safety and effectiveness of medicines and vaccines

Facilitate the advancement and application of novel health technologies and methodologies

Build international partnerships aiding public and private organisations to fulfil their scientific potential
Provide quality management consultancy to comply with the pharmacovigilance standards for medicines and vaccines research
We work according to research management best practices through a quality management system that:

Meets medical research quality requirements

Increases process efficiency

Ensures regulatory compliance
OUR SERVICES
Scientific management of pharmacoepidemiology, safety and effectiveness studies of medicines and vaccines based on real-world evidence
Post-authorisation safety studies
Post-authorisation effectiveness studies
Medicines and vaccines utilisation
Guidance, management, and scientific support to EMA regulatory procedures at different stages of medicines/therapies, devices, and methodologies development

EMA Scientific Advice and protocol assistance
Qualification of novel methodologies for medicine development
Innovation Task Force briefing meeting
Expertise in the coordination, governance and business development of multistakeholder international networks dedicated to pharmacoepidemiology
VAC4EU
(Vaccine monitoring collaboration for Europe)
International network of multi-stakeholders that promotes real world evidence creation on vaccine coverage, safety, and effectiveness. VAC4EU has 24 members, from 9 European countries, and 17 data access providers covering an overall population of 152 million.
OUR NUMBERS

We are actively conducting 11 Pharmacoepidemiology and Real-Word Evidence studies, and have successfully finalised 3.


Our work has resulted in the generation of 11 publications, furthering the understanding of Pharmacoepidemiology and Real-World Evidence

1 Qualification of novel methodologies for medicine development

3 Innovation Task Force briefing meetings

OUR NETWORK
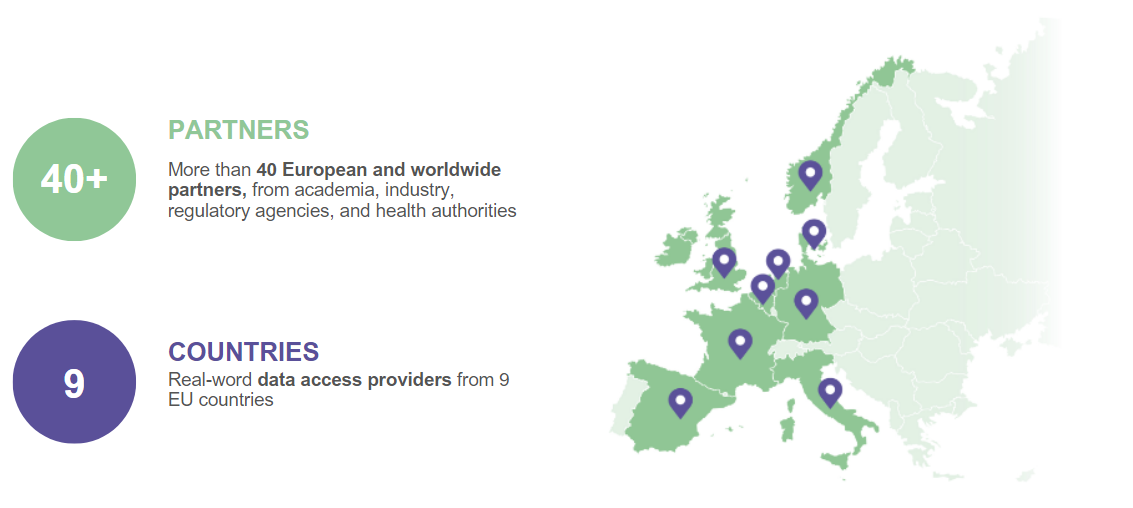

Where we are
Barcelona Health Hub
Recinte Modernista de Sant Pau
c/ Sant Antoni María Claret, 167
08025 Barcelona (Spain)
Get in touch!
T +34 930 180 163
info@teamitresearch.com

is a spinoff of
